
TALVEY® is the first and only FDA-approved bispecific antibody developed to target GPRC5D1,2
The heterogeneous nature of multiple myeloma highlights the need for new treatment targets3
Different targets may help address treatment resistance and avoid reexposure to previous targets4–6
- With emerging research, GPRC5D has been identified as a target with a potential role in cancer treatment5,6
- GPRC5D is expressed on the surface of multiple myeloma cells and non-malignant plasma cells. It is also expressed on healthy tissues such as epithelial cells in keratinized tissues of the skin and tongue1,5,7–10
- Expressed in a broad range of patients with multiple myeloma that varied according to disease staging, cytogenetic abnormalities, gender, and age5
- Expression is independent of other targets, including BCMA6
GPRC5D expression has limited to no known impact on healthy B cells, and is prominently found on malignant multiple myeloma cells5,9–11
GPRC5D expression is not detected in early B cell lines, such as pro-B cells and early progenitor cell lines9
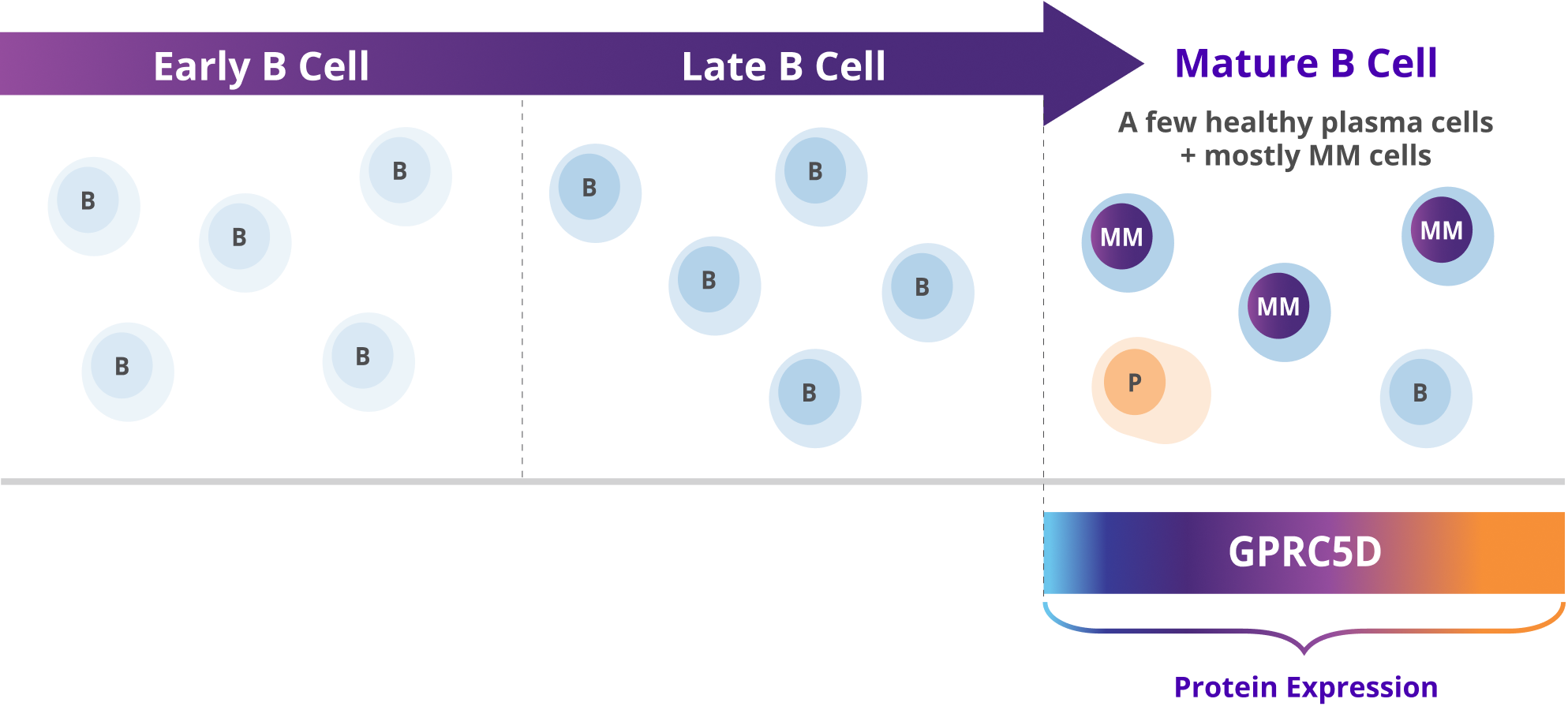
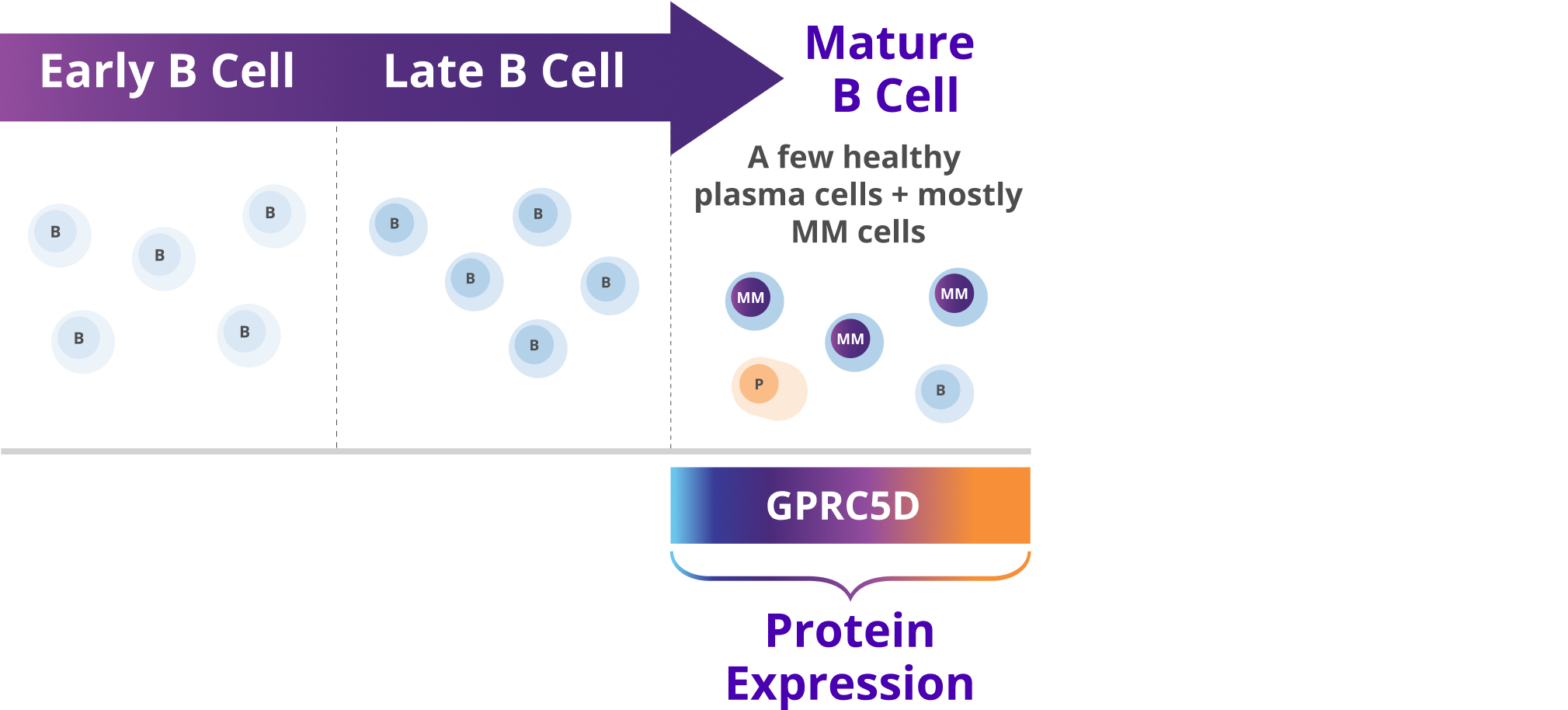
B, B cells; BCMA, B cell maturation antigen; FDA, U.S. Food and Drug Administration; GPRC5D, G protein-coupled receptor class C group 5 member D; MM, multiple myeloma; MOA, mechanism of action; P, plasma cells.
- TALVEY® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- U.S. FDA approves TALVEY® (talquetamab-tgvs), a first-in-class bispecific therapy for the treatment of patients with heavily pretreated multiple myeloma. Janssen Biotech, Inc. Updated August 10, 2023. Accessed June 24, 2024. https://www.janssen.com/fda-approves-talveytm-talquetamab-tgvs-first-class-bispecific-therapy-treatment-patients-heavily
- Kurtin SE. Relapsed or relapsed/refractory multiple myeloma. J Adv Pract Oncol. 2013;4(suppl 1):5–14.
- Mateos MV, Weisel K, De Stefano V, et al. LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma. Leukemia. 2022;36(5):1371–1376.
- Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur J Clin Invest. 2012;42(9):953–960.
- Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746.
- Inoue S, Nambu T, Shimomura T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Invest Dermatol. 2004;122(3):565–573.
- Lancman G, Sastow DL, Cho HJ, et al. Bispecific antibodies in multiple myeloma: present and future. Blood Cancer Discov. 2021;2(5):423–433.
- Kodama T, Kochi Y, Nakai W, et al. Anti-GPRC5D/CD3 bispecific T-cell-redirecting antibody for the treatment of multiple myeloma. Mol Cancer Ther. 2019;18(9):1555–1564.
- Verkeij CPM, Broekmans MEC, van Duin M, et al. Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv. 2021;5(8):2196–2215.
- Hammons L, Szabo A, Janardan A, et al. The changing spectrum of infection with BCMA and GRPC5D targeting bispecific antibody (bsAb) therapy in patients with relapsed refractory multiple myeloma. Haematol. 2024;109(3):906-914.
- Bräuner-Osborne H, Jensen AA, Sheppard PO, Brodin B, Krogsgaard-Larsen P, O'Hara P. Cloning and characterization of a human orphan family C G-protein coupled receptor GPRC5D. Biochim Biophys Acta. 2001;1518(3):237–248.
TALVEY® induces the lysis of multiple myeloma cells by activating the immune system via GPRC5D × CD31
- In research that examined GPRC5D mRNA expression in malignant cells, GPRC5D mRNA was found to be substantially expressed in multiple myeloma cell lines3
- This finding led to the commitment of Janssen Biotech, Inc., to pursue the development of a GPRC5D-targeting therapy for multiple myeloma4


This video illustrates the MOA of TALVEY®
For an overview of the MOA of TALVEY® and the role GPRC5D plays in multiple myeloma
Get ResourceCD, cluster of differentiation; FDA, U.S. Food and Drug Administration; GPRC5D, G protein-coupled receptor class C group 5 member D; MOA, mechanism of action; mRNA, messenger ribonucleic acid.
- TALVEY® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- U.S. FDA approves TALVEY® (talquetamab-tgvs), a first-in-class bispecific therapy for the treatment of patients with heavily pretreated multiple myeloma. News release. Janssen Biotech, Inc.; August 10, 2023. Accessed June 24, 2024. https://www.janssen.com/fda-approves-talveytm-talquetamab-tgvs-first-class-bispecific-therapy-treatment-patients-heavily
- Smith EL, Harrington K, Staehr M, et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci Transl Med. 2019;11(485):eaau7746.
- Pillarisetti K, Edavettal S, Mendonça M, et al. A T-cell- redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135(15):1232–1243.
