
MonumenTAL-1 longer-term follow-up analysis
View findings for patients exposed to T-cell redirection therapy >
MonumenTAL-1 longer-term follow-up analysis at a median follow-up of >23 months in patients naïve to T-cell redirection therapy1*
You are now viewing a subsequent follow-up analysis of the MonumenTAL-1 trial. This information is not included in the current full Prescribing Information. These long-term follow-up data reflect the patients naïve to TCR therapy* receiving TALVEY® Q2W; any increase in n-value is due to this longer-term follow-up and additional patients.
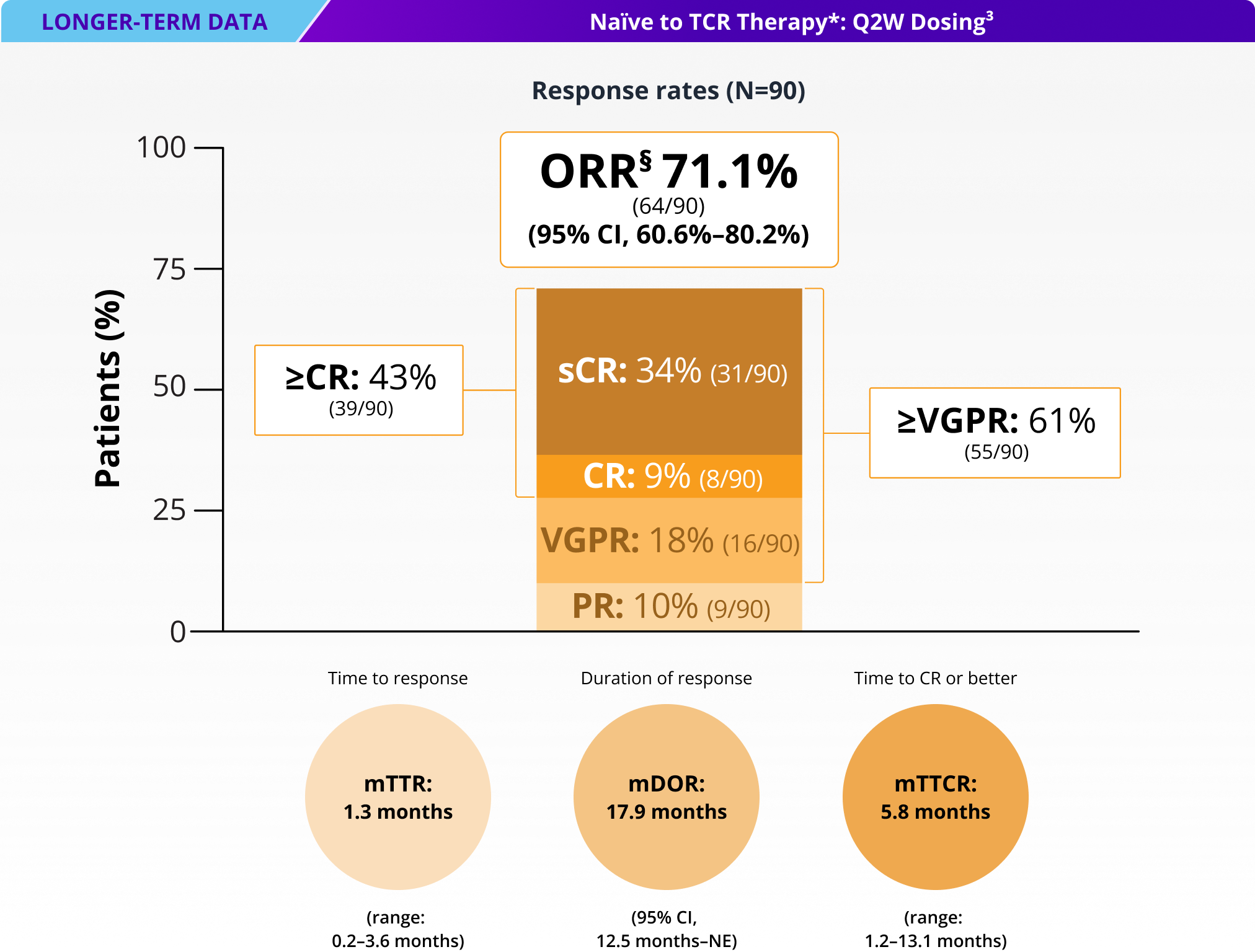
71% of patients responded to TALVEY® Q2W dosing, with 43% achieving ≥CR†
ORR and DOR as assessed by an IRC using IMWG criteria.2‡


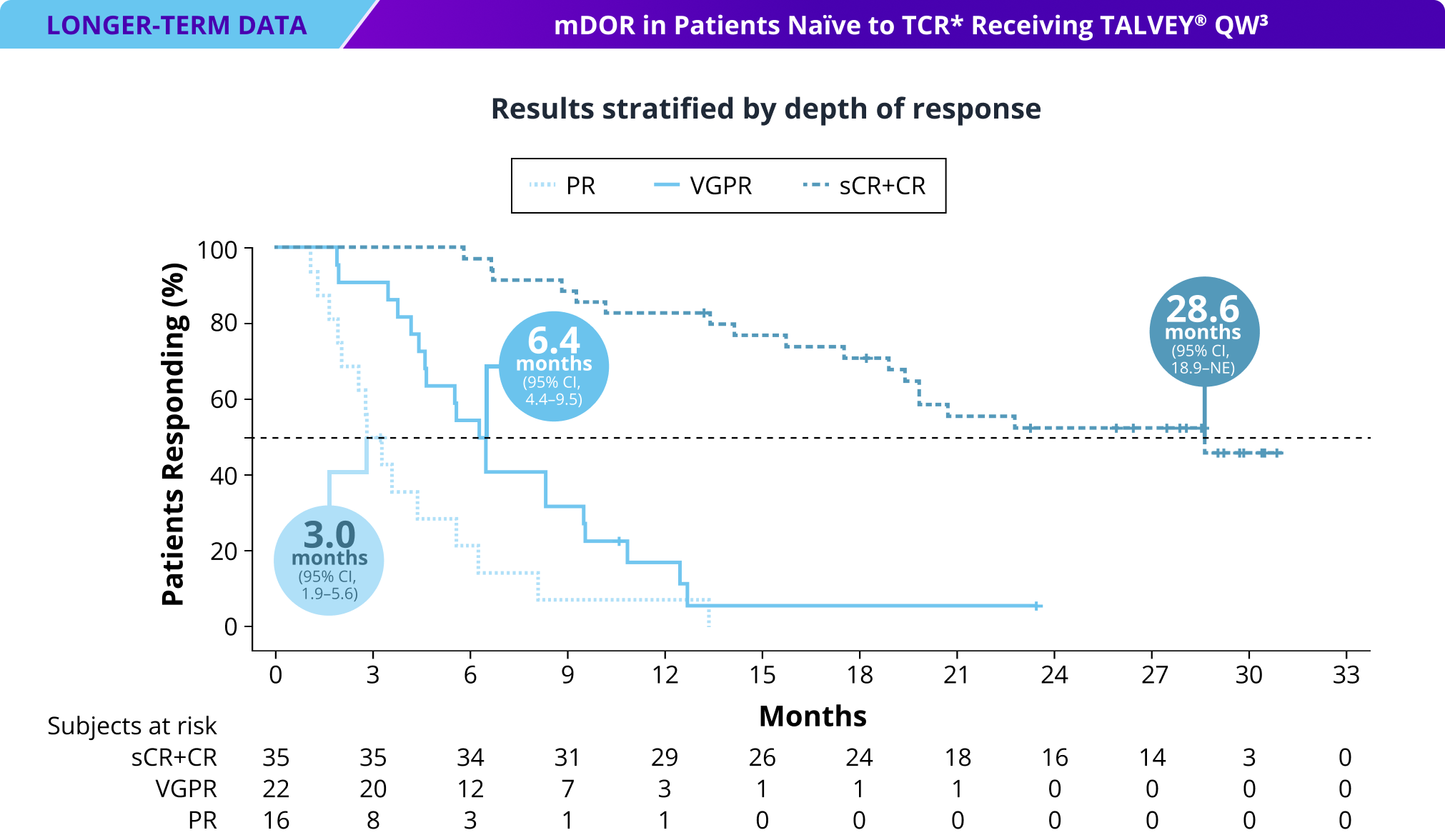
Long-term DOR at median follow-up of >23 months in patients naïve to T-cell redirection therapy* receiving TALVEY® Q2W1



LONGER-TERM DATA
mDOR for total Q2W TCR-naïve population: 17.9 months (95% CI, 12.5 months–NE)3
MonumenTAL-1 longer-term follow-up analysis at a median follow-up of >29 months in patients naïve to T-cell redirection therapy1*
You are now viewing a subsequent follow-up analysis of the MonumenTAL-1 trial. This information is not included in the current full Prescribing Information. These long-term follow-up data reflect the patients naïve to TCR therapy* receiving TALVEY® QW.
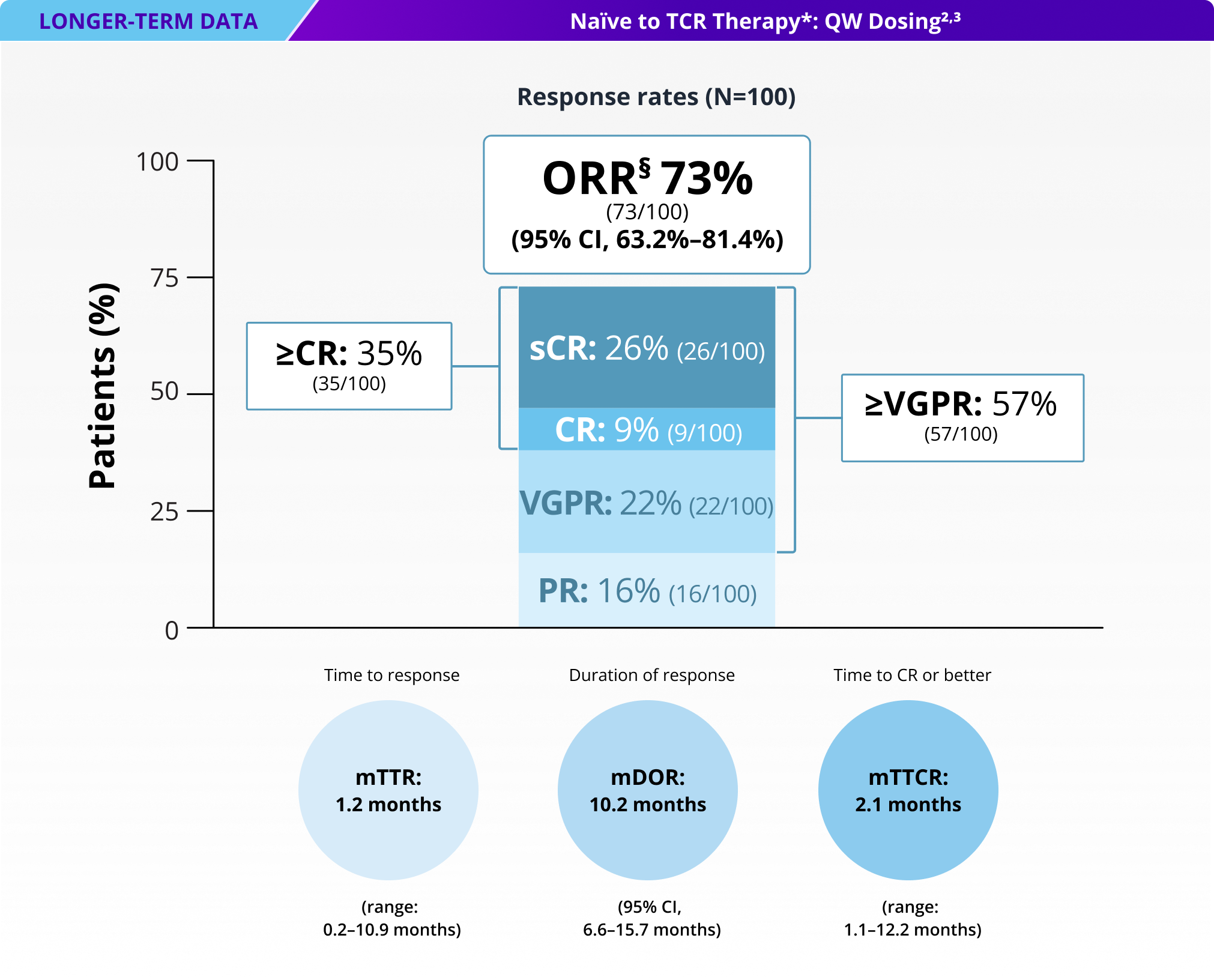
73% of patients responded to TALVEY® QW dosing, with 35% achieving ≥CR†
ORR and DOR as assessed by an IRC using IMWG criteria.2‡


Long-term DOR at median follow-up of >29 months in patients naïve to T-cell redirection therapy* receiving TALVEY® QW1


LONGER-TERM DATA
mDOR for total TCR-naïve QW population: 10.2 months (95% CI, 6.6–15.7 months)3
MonumenTAL-1 longer-term follow-up analysis at a median follow-up of >20 months in patients exposed to T-cell redirection therapy1*
You are now viewing a subsequent follow-up analysis of the MonumenTAL-1 trial. This information is not included in the current full Prescribing Information. These long-term follow-up data reflect the patients exposed to TCR therapy* receiving TALVEY® QW; any increase in n-value is due to this longer-term follow-up and additional patients.
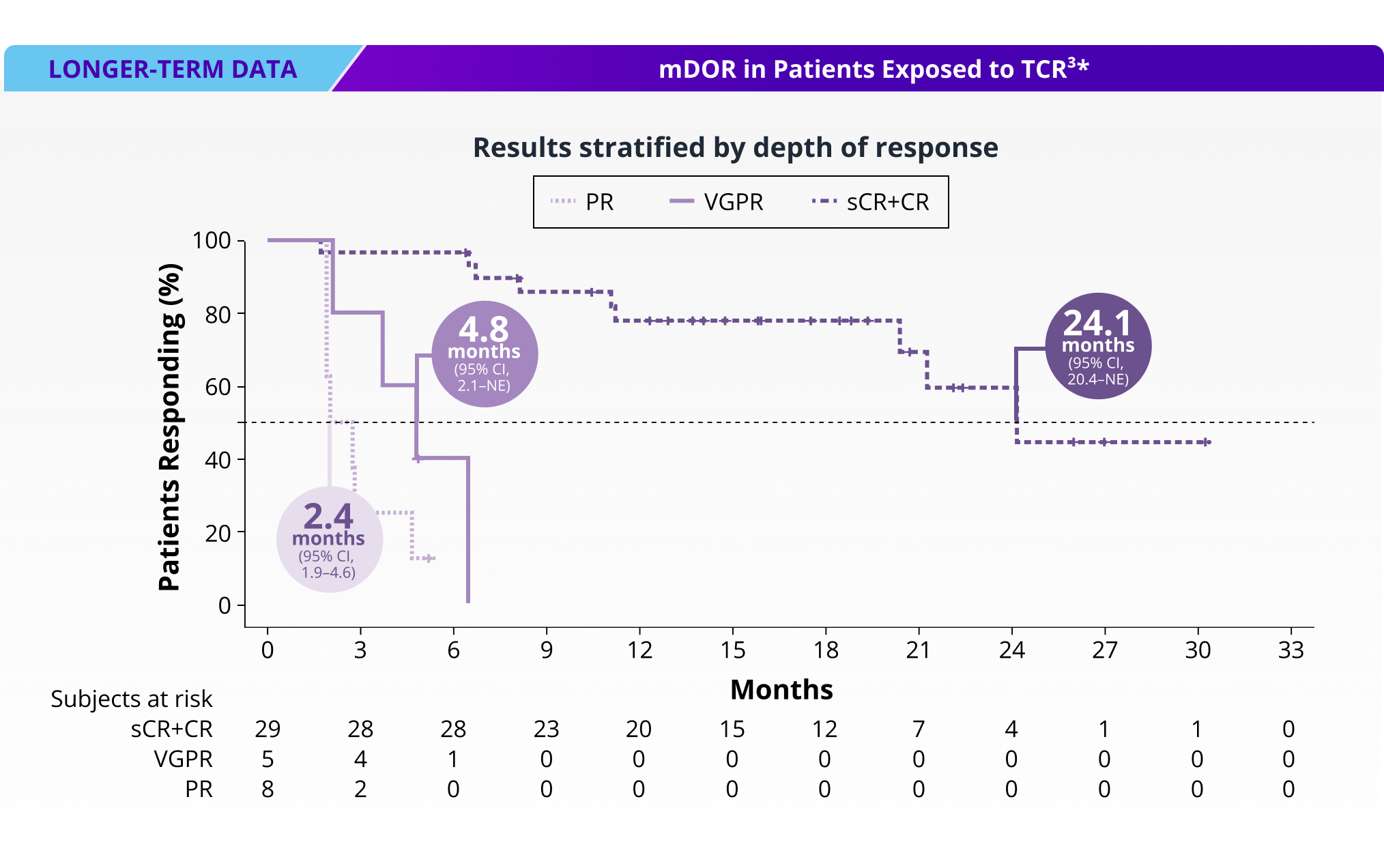
72% of patients responded to TALVEY®, with 50% achieving ≥CR3†



Long-term DOR at median follow-up of >20 months in patients exposed to T-cell redirection therapy1*


LONGER-TERM DATA
mDOR for total TCR-exposed population: 21.3 months (95% CI, 6.7 months–NE)3
*T-cell redirection therapy refers to both CAR-T and bispecific antibody therapy.
†≥CR: sCR+CR.
‡Efficacy results reflect patients who have received ≥4 prior lines of therapy.
§ORR: sCR+CR+PR+VGPR.
BCMA, B-cell maturation agent; CAR-T, chimeric antigen receptor T-cell; CI, confidence interval; CR, complete response; LTFU, longer-term follow-up; mDOR, median duration of response; mTTCR, median time to CR; mTTR, median time to response; NE, not estimable; ORR, overall response rate; PR, partial response; QW, once weekly; Q2W, every 2 weeks; sCR, stringent complete response; TCR, T-cell redirection; VGPR, very good partial response.
- Rasche L, Schicke C, Touzeau C, et al. Long-term efficacy and safety results from phase 1/2 MonumenTAL-1 Study of talquetamab, a GPRC5DxCD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Poster. Presented at the European Hematology Association (EHA) 2024 Hybrid Congress; June 13-16, 2024; Madrid, Spain.
- TALVEY® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Data on file. Janssen Biotech, Inc.
Longer-term follow-up safety analysis1
You are now viewing a subsequent follow-up analysis of the MonumenTAL-1 trial. This information is not included in the current full Prescribing Information.
Adverse reactions (≥20%) in patients with relapsed or refractory multiple myeloma who received TALVEY® in the MonumenTAL-1 longer-term follow-up analysis*
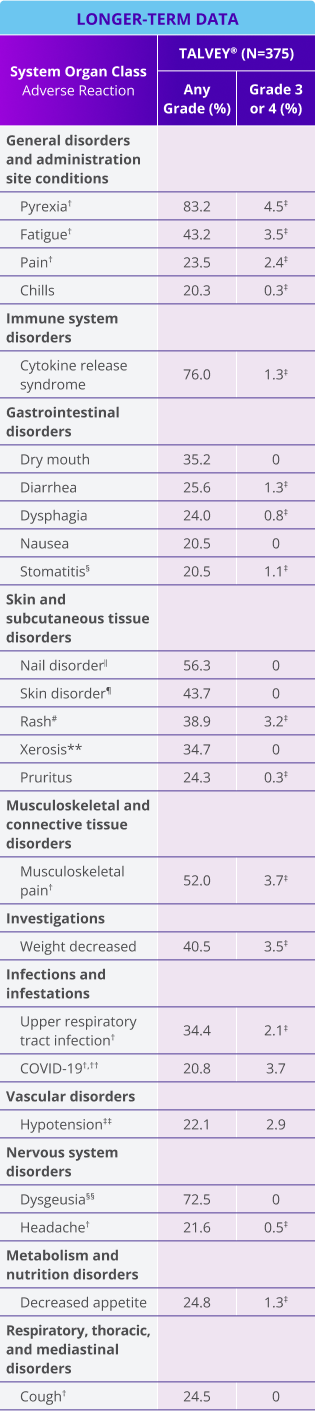
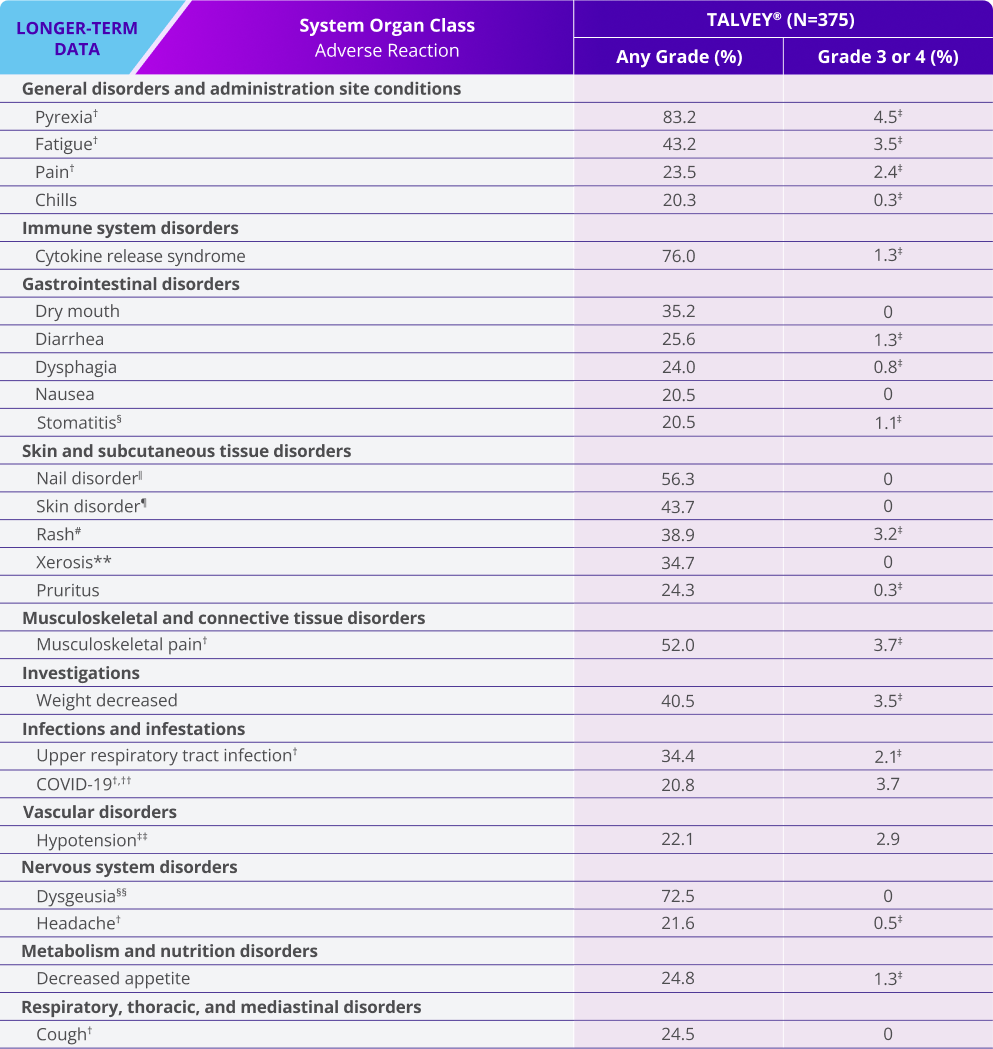
LONGER-TERM DATA
6.9% of patients discontinued TALVEY® due to an adverse reaction
Updated discontinuation rate reflects longer-term follow-up* data of MonumenTAL-1 cohorts with a total N value of 375.2
Note: CRS was originally graded by Lee criteria (Lee et al. 2014) in phase 1 and by ASTCT consensus grading system (Lee et al 2019) in phase 2, with conversion of grade in phase 1 to ASTCT based on data in eCRF. Toxicity grade by ASTCT is presented in this table, for both phase 1 and phase 2.
Note: Adverse events are reported until 100 days (phase 1) or 30 days (phase 2) after the last dose of talquetamab or until the start of subsequent anticancer therapy, if earlier.
Note: The output includes the diagnosis of CRS; the symptoms of CRS are included.
Note: Subjects are counted only once for any given event, regardless of the number of times they actually experienced the event. Adverse events are coded using MedDRA Version 24.1.
*Median follow-up for MonumenTAL-1 cohorts: TCR-naïve Q2W is over 23 months; TCR-exposed is over 20 months; TCR-naïve QW is over 29 months.
†Includes other related terms.
‡Only Grade 3 adverse reactions occurred.
§Stomatitis includes: cheilitis, glossitis, glossodynia, mouth ulceration, oral discomfort, oral mucosal erythema, oral pain, stomatitis, swollen tongue, tongue discomfort, tongue erythema, tongue edema, and tongue ulceration.
‖Nail disorder includes: koilonychia, nail bed disorder, nail cuticle fissure, nail discoloration, nail disorder, nail dystrophy, nail hypertrophy, nail pitting, nail ridging, nail toxicity, onychoclasis, onycholysis, and onychomadesis.
¶Skin disorder includes: palmar-plantar erythrodysesthesia syndrome, palmoplantar keratoderma, skin discoloration, skin exfoliation, and skin fissures.
#Rash includes: dermatitis, dermatitis acneiform, dermatitis contact, dermatitis exfoliative, dermatitis exfoliative generalized, erythema, exfoliative rash, rash, rash erythematous, rash macular, rash maculopapular, rash popular, rash pruritic, rash pustular, rash vesicular, and stasis dermatitis.
**Xerosis includes: dry eyes, dry skin, and xerosis.
††Contains fatal outcome(s).
‡‡Hypotension includes: hypotension and orthostatic hypotension.
§§Dysgeusia includes: ageusia, dysgeusia, hypogeusia, and taste disorder.
AR, adverse reaction; ASTCT, American Society for Transplantation and Cellular Therapy; COVID, coronavirus disease; CRS, cytokine release syndrome; eCRF, electronic case report form; MedDRA, Medical Dictionary for Regulatory Activities; Q2W, every 2 weeks; QW, once weekly; TCR, T-cell redirection.
Oral, skin, and nail toxicity and weight-related ARs seen with TALVEY® in the longer-term follow-up analysis1,2*
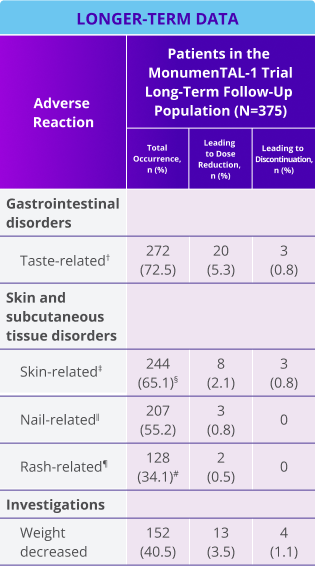
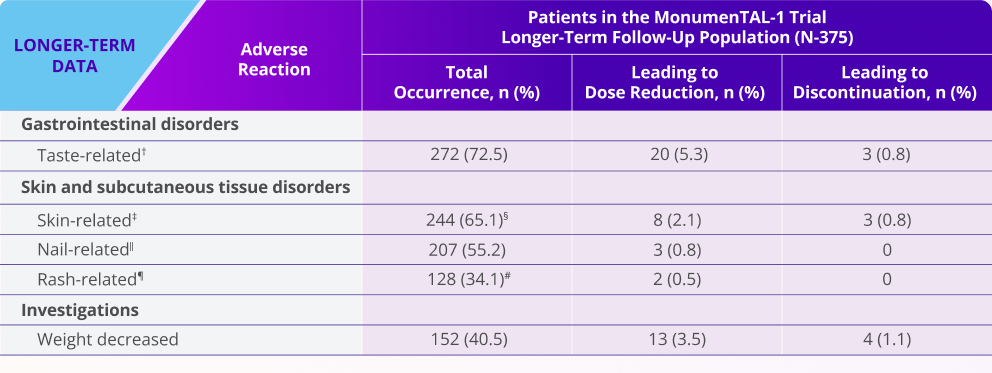
Note: Dose reduction could mean increasing the interval between doses and/or reducing the overall dose.
*Median follow-up for MonumenTAL-1 cohorts: TCR-naïve Q2W is over 23 months, TCR-exposed is over 20 months, TCR-naïve QW is over 29 months.
†Includes ageusia, dysgeusia, hypogeusia, and taste disorder.
‡Includes skin exfoliation, dry skin, pruritus, and palmar-plantar erythrodysesthesia syndrome.
§Includes 1 (0.6%) grade ¾ event for patients naïve to T-cell redirection therapy receiving TALVEY® Q2W.
‖Includes nail discoloration, nail disorder, onycholysis, onychomadesis, onychoclasis, nail dystrophy, nail toxicity, and nail ridging.
¶Includes rash, maculopapular rash, erythematous rash, and erythema.
#Includes 12 (3.2%) grade ¾ events.
TALVEY® is associated with a low rate of Grade 3/4 infections (9.3%), death due to infections (1.3%), and discontinuation due to infections (0.8%)1*
Occurrence, discontinuation, and death due to infection in patients receiving TALVEY®
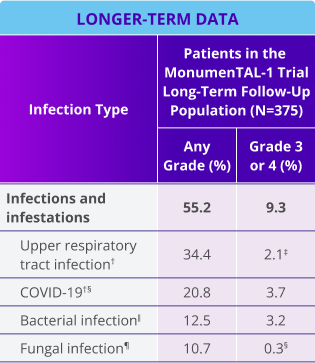

Death due to infection
Discontinuation due to infection
*Median follow-up for MonumenTAL-1 cohorts: TCR-naïve Q2W is over 23 months; TCR-exposed is over 20 months; TCR-naïve QW is over 29 months.
†Includes other related terms.
‡Only Grade 3 adverse reactions occurred.
§Contains fatal outcome(s).
‖Bacterial infection includes: campylobacter infection, carbuncle, cellulitis, citrobacter infection, clostridium difficile colitis, clostridium difficile infection, cystitis escherichia, cystitis klebsiella, diverticulitis, escherichia pyelonephritis, folliculitis, gastroenteritis escherichia coli, helicobacter gastritis, human ehrlichiosis, impetigo, klebsiella sepsis, moraxella infection, otitis media acute, pitted keratolysis, pseudomonal bacteremia, pyuria, relapsing fever, renal abscess, skin infection, staphylococcal infection, tooth abscess, tooth infection, urinary tract infection enterococcal, and urinary tract infection pseudomonal.
¶Fungal infection includes: body tinea, candida infection, fungal foot infection, fungal infection, fungal skin infection, genital candidiasis, esophageal candidiasis, onychomycosis, oral candidiasis, oral fungal infection, oropharyngeal candidiasis, tinea pedis, vulvovaginal candidiasis, and vulvovaginal mycotic infection.
Incidence of Grade 3+ infections and IgG levels over time1*
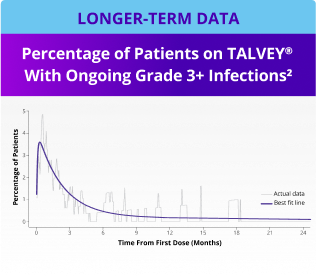
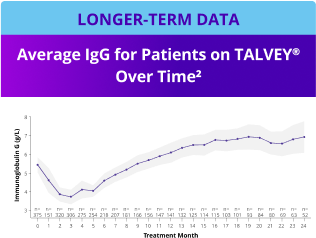
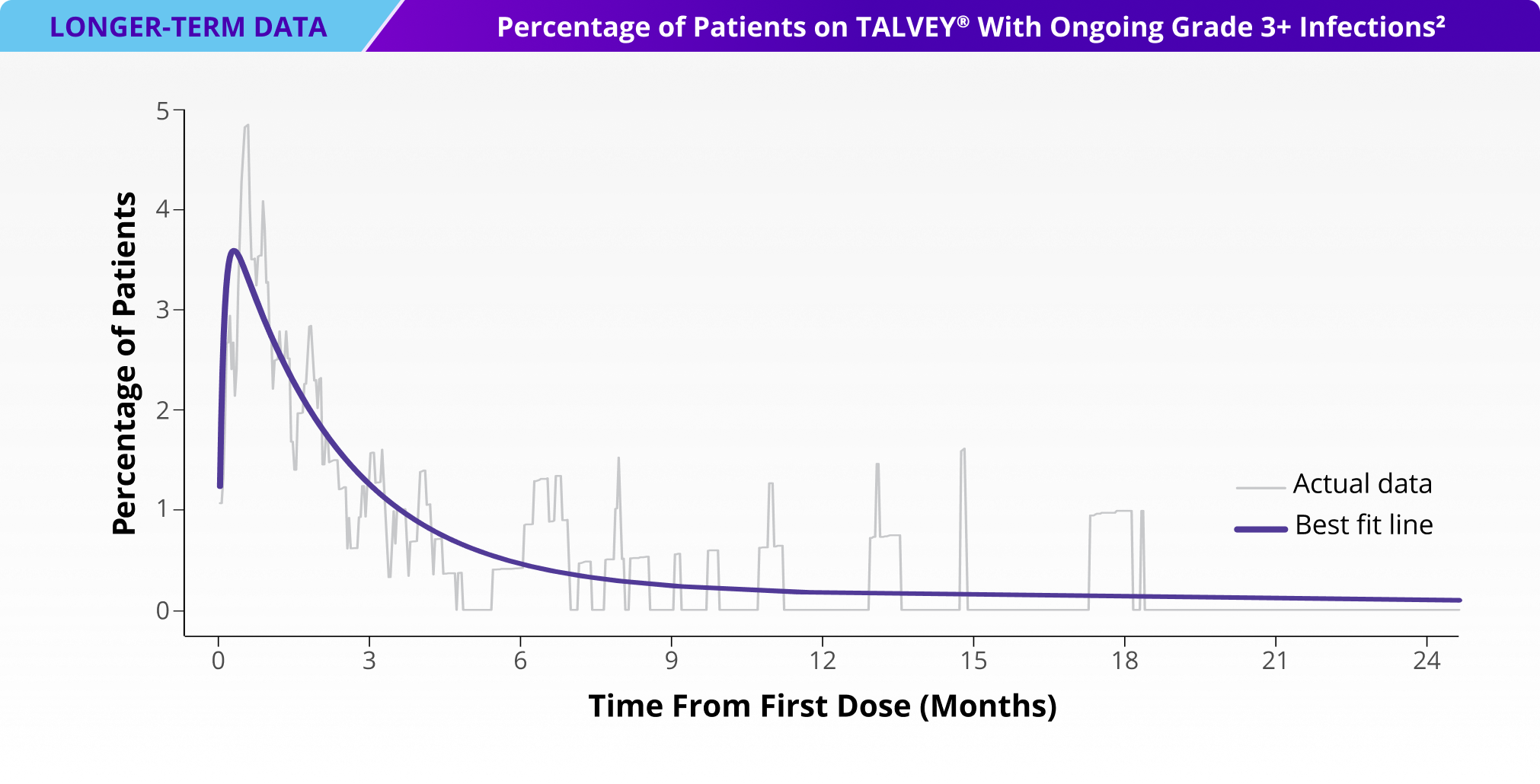
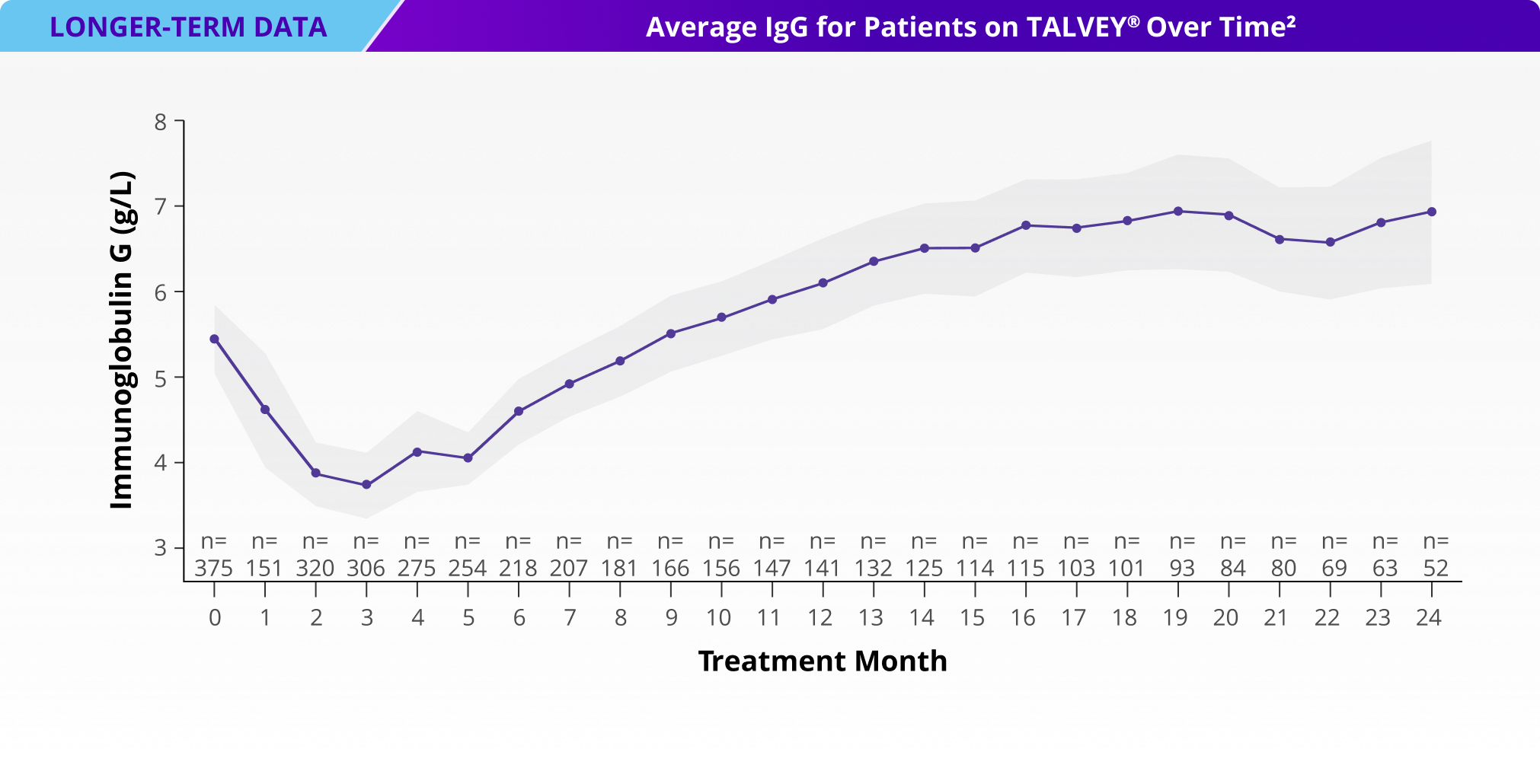
*Median follow-up for MonumenTAL-1 cohorts: TCR-naïve Q2W is over 23 months; TCR-exposed is over 20 months; TCR-naïve QW is over 29 months.
IgG, immunoglobulin G.
LTFU, longer-term follow-up.
- Data on file. Janssen Biotech, Inc.
- Rasche L, Schicke C, Touzeau C, et al. Long-term efficacy and safety results from phase 1/2 MonumenTAL-1 Study of talquetamab, a GPRC5DxCD3 bispecific antibody, in patients with relapsed/refractory multiple myeloma. Poster. Presented at the European Hematology Association (EHA) 2024 Hybrid Congress; June 13-16, 2024; Madrid, Spain.
