Patients (%)
ORR¶ 72%
(23/32)
(95% CI, 53%–86%)
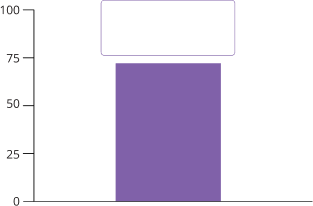
Median follow-up of 10.4 months
With a median follow-up of 10.4 months, an estimated
59% of patients continued to respond for
at least 9 months.

In patients who have been triple-class exposed, TALVEY® provided
The efficacy of TALVEY® as a single agent was evaluated in 219 patients with relapsed or refractory multiple myeloma in the single-arm, open-label, multicenter, phase 1/2 MonumenTAL-1 trial.1–3
Patients naïve to T-cell redirection therapy* were randomized to receive TALVEY® Q2W or QW:
(N=87)
(N=100)
Patients exposed to T-cell redirection therapy* received TALVEY® QW:
(N=32)
ORR3
Key secondary endpoints: DOR and TTR3
Patients received TALVEY® Q2W (0.8 mg/kg) or QW (0.4 mg/kg) as a subcutaneous injection until disease progression or unacceptable toxicity, after the step-up dosing schedule.
MonumenTAL-1 is the only clinical trial that included patients with ADC exposure and a specific cohort of patients with prior TCR exposure (bispecific antibody and/or CAR-T cell therapy)
*T-cell redirection therapy refers to both CAR-T and bispecific antibody therapy.
ADC, antibody-drug conjugates; CAR-T, chimeric antigen receptor-T cell; CD, cluster of differentiation; CNS, central nervous system; CRS, cytokine release syndrome; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; ORR, overall response rate; QW, once weekly; Q2W, every 2 weeks; TCR, T-cell receptor; TTR, time to response.
| Naïve to T-Cell Redirection Therapy* | |
|---|---|
| Patient Characteristics | SC Q2W/QW (N=187) |
| Age, median | 67 years (range: 38–86) |
| Gender Male | 57% |
| Race White Hispanic Black or African American Asian | 90% 8% 5% 3% |
| ISS stage I II III | 44% 34% 22% |
| High-risk cytogenetics (presence of t[4;14], t[14;16], and/or del[17p])† | 29% |
| Extramedullary disease | 22% |
| Prior lines of therapy, median | 5 lines (range: 4–13) |
| Prior autologous stem cell transplantation | 78% |
| Triple-class exposed (proteasome inhibitor, immunomodulatory agent, and anti-CD38 monoclonal antibody) | 100% |
| Triple-class refractory (proteasome inhibitor, immunomodulatory agent, and anti-CD38 monoclonal antibody) | 73% |
| Refractory to last therapy | 94% |
In patients exposed to T-cell redirection therapy,* 81% had prior CAR-T and 25% had prior bispecific antibody therapy
| Exposed to T-Cell Redirection Therapy* | |
|---|---|
| Patient Characteristics | SC QW (N=32) |
| Prior lines of therapy, median | 6 lines (range: 4–15) |
| Triple-class exposed (proteasome inhibitor, immunomodulatory agent, and anti-CD38 monoclonal antibody) | 100% |
| Prior CAR-T therapy | 81% |
| Prior bispecific antibody therapy | 25% |
| Prior BCMA-directed therapy | 94% |
*T-cell redirection therapy refers to both CAR-T and bispecific antibody therapy.
†Baseline cytogenetic data were not available in 11% of patients.
BCMA, B-cell maturation antigen; CAR-T, chimeric antigen receptor-T cell; CD, cluster of differentiation; del(17p), deletion 17p; ISS, International Staging System; QW, once weekly; Q2W, every 2 weeks; SC, subcutaneous; t, translocation.
Efficacy was based on ORR and DOR as assessed by an IRC using IMWG criteria.1*

Median follow-up of 5.9 months (range: 0–9.5) from first response
Median follow-up of 13.8 months (range: 0.8–15.4) from first response
Patients who received TALVEY® achieved durable responses
Responses with Q2W dosing (N=87)
mTTR:
1.3 months
(range: 0.2–9.2 months)
mDOR:
NE
(range: 0.2–9.2 months)
Median duration of follow-up from first response among responders was 5.9 months.
An estimated 85% of patients continued to respond for at least 9 months.
Responses with QW dosing (N=100)
mTTR:
1.2 months
(range: 0.2–10.9 months)
mDOR:
9.5 months
(95% CI, 6.5–NE months)
Median duration of follow-up from first response among responders was 5.9 months;
an estimated 85% of patients continued to respondddd for at least 9 months.
Naïve to T-Cell Redirection Therapy1,2†
About 73% of patients responded to TALVEY®, with ≥32% achieving ≥CR‡
Median prior lines of therapy: 5 (range: 4–13)§
Deep responses with Q2W dosing (N=87)||

Median follow-up of 5.9 months (range: 0–9.5) from first response
Deep responses with QW dosing (N=100)||

Median follow-up of 13.8 months (range: 0.8–15.4) from first response
Patients who received TALVEY® achieved durable responses
Responses with Q2W dosing (N=87)
mTTR:
1.3 months
(range: 0.2–9.2 months)
mDOR:
NE
(range: 0.2–9.2 months)
Median duration of follow-up from first response among responders was 5.9 months;
an estimated 85% of patients continued to respond for at least 9 months.
Responses with QW dosing (N=100)
mTTR:
1.2 months
(range: 0.2–10.9 months)
mDOR:
9.5 months
(95% CI, 6.5–NE months)
Median duration of follow-up from first response among responders was 5.9 months;
an estimated 85% of patients continued to respond for at least 9 months.
(range: 0.2–9.2 months)
T-cell redirection therapy refers to both CAR-T and bispecific antibody treatment.
≥CR: sCR+CR
Reflects the median prior lines of therapy for the entire naïve to T-cell redirection therapy population (Q2W and QW dosing).
§Reflects the median prior lines of therapy for the entire naïve to T-cell redirection therapy population (Q2W and QW dosing).
Deep responses: sCR+CR+VGPR.
ORR: sCR+CR+VGPR+PR.
Due to rounding, calculation may not be exact.
CR, complete response; mDOR, median duration of response; mTTR, median time to response; ORR, overall response rate; sCR, stringent complete response; TCR, T-cell redirection; VGPR, very good partial response; QW, once weekly; Q2W, every 2 weeks.
Efficacy was based on ORR and DOR as assessed by an IRC using IMWG criteria.1*
Exposed to T-Cell Redirection Therapy†
Responses with QW dosing (N=32)
Median prior therapies: 6 (range: 4–15)
Response rates
Patients (%)
ORR¶ 72%
(23/32)
(95% CI, 53%–86%)

Median follow-up of 10.4 months
With a median follow-up of 10.4 months, an estimated
59% of patients continued to respond for
at least 9 months.
Exposed to T-Cell Redirection Therapy†
Responses with QW dosing (N=32)
Median prior therapies: 6 (range: 4–15)
Response rates
Patients (%)
ORR¶ 72%
(23/32)
(95% CI, 53%–86%)

Median follow-up of 10.4 months
With a median follow-up of 10.4 months, an estimated
59% of patients continued to respond for at least 9 months
*Efficacy results reflect patients who received ≥4 prior lines of therapy.
T-cell redirection therapy refers to both CAR-T and bispecific antibody therapy.
≥CR: sCR+CR.
§Reflects the median prior lines of therapy for the entire naïve to T-cell redirection therapy population (Q2W and QW dosing).
‖Deep responses: sCR+CR+VGPR.
ORR: sCR+CR+VGPR+PR.
Due to rounding, calculation may not be exact.
CAR-T, chimeric antigen receptor-T cell; CI, confidence interval; CR, complete response; DOR, duration of response; IMWG, International Myeloma Working Group; IRC, Independent Review Committee; mDOR, median duration of response; mTTR, median time to response; NE, not estimable; ORR, overall response rate; PR, partial response; QW, once weekly; Q2W, every 2 weeks; sCR, stringent complete response; TCR, T-cell redirection; VGPR, very good partial response.